On 1 February 2025, new regulations limiting the pack sizes of paracetamol-containing products came into effect across Australia. Announced by the Therapeutic Goods Administration (TGA) on 3 May 2024, these changes are aimed at reducing the risk of harm from intentional overdose while ensuring continued access for pain relief.
The move brings Australia into line with international efforts such as in the UK to reduce intentional misuse of paracetamol. Pack sizes were reduced in the UK in the late 1990s and follow-up research shows that the legislative changes have been followed by significant reductions in deaths due to paracetamol overdose.
Key changes from 1 Feb 2025
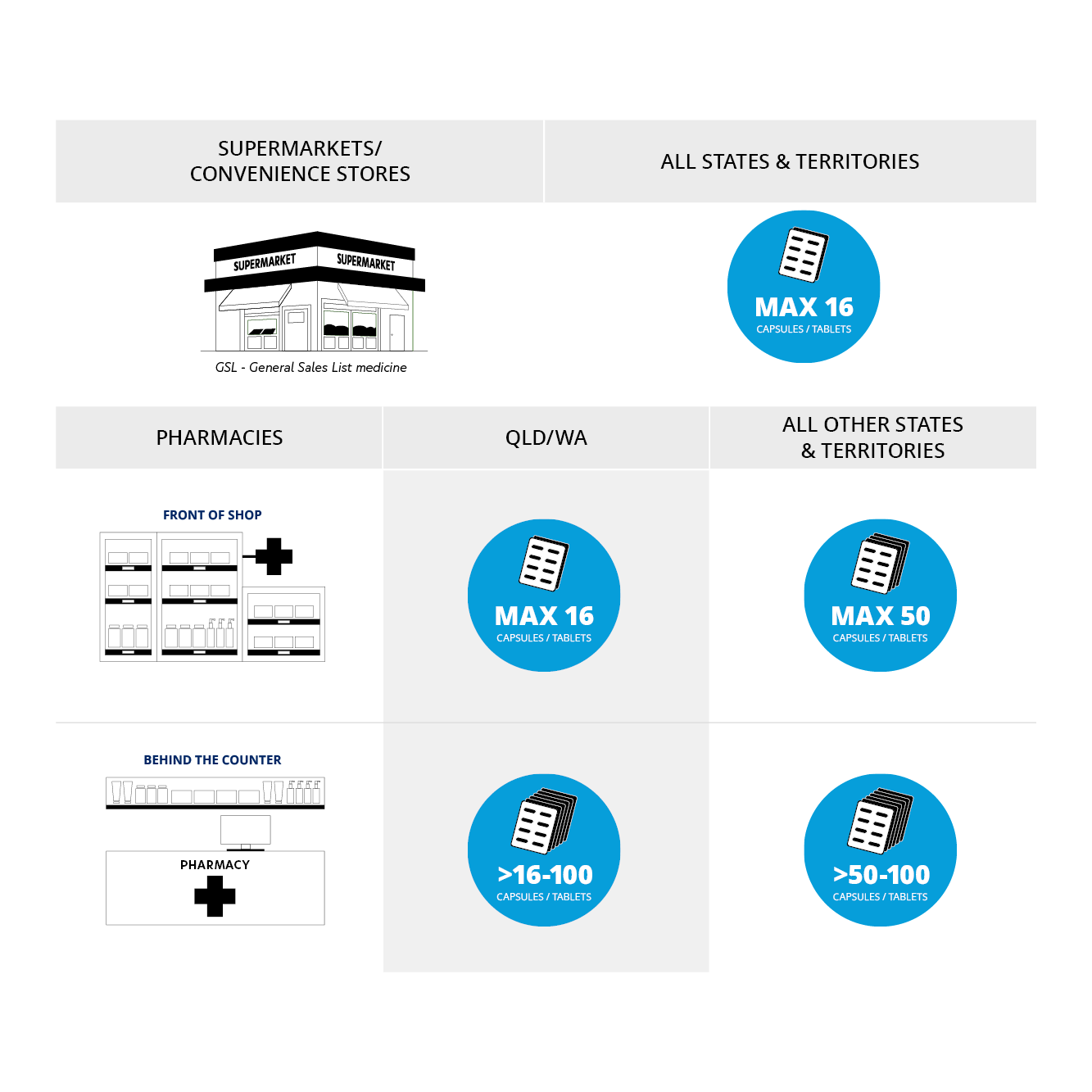
How paracetamol-containing products can be sold depends on how many tablets or capsules are in the package, where you are purchasing them and where you are located in Australia. Listen to National President Professor Trent Twomey as he explains the changes in a conversation on Weekend Breakfast.
Non pharmacy retail
All paracetamol-containing products sold in retail outlets such as supermarkets and grocery stores must now be in blister packaging. The maximum number of tablets or capsules that can be sold in this setting has been reduced from 20 to 16 tablets or capsules.
Pharmacy
In pharmacies, paracetamol-containing products are now scheduled as either Schedule 2 (S2) or Schedule 3 (S3) depending on how many tablets or capsules are in the pack.
Packs of up to 50 are now classified as S2. In all states and territories except Western Australia and Queensland, S2 medicines can be displayed on a shelf in the front shop area. In Queensland and Western Australia however, S2 products must be kept behind the counter.
Packs of 51-100 are now classified as S3.
In all states and territories—including Western Australia and Queensland—packs containing more than 50 tablets or capsules must now be kept behind the counter as Schedule 3 Pharmacist-Only medicine. That means they require a pharmacist’s oversight for sale. While not mandatory to be sold in blister packs, it is preferred, however it is ultimately at the pharmacist’s discretion and in some situations a bottle may be used, for example for a patient with dexterity issues.
A summary of the changes is pictured below:
There is no change to the suitability profile and efficacy for paracetamol for pain relief when used as directed.
Additionally, there are no changes to:
- Treatment guidelines or recommendations for use as a result of the scheduling change.
- Advertising regulations of paracetamol-containing products.
- Patients should be encouraged to only buy what they need and not stockpile paracetamol-containing products in the home. Pharmacists will use their professional discretion to supply what is needed. Principally, paracetamol-containing products and other medicines should be stored in a secure location in the home, out of sight and reach of children.
- Oral liquid and modified release paracetamol products.
To assist the industry transition the TGA has provided a 12-month period of labelling exemption. Full details can be found on the TGA website: Temporary labelling exemptions for paracetamol | Therapeutic Goods Administration (TGA).
For member pharmacies, the Guild has prepared a poster for display in the pharmacy to advise patients of the changes.


